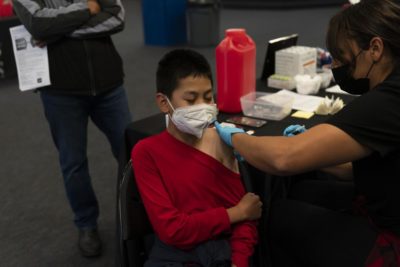
UNIVERSITY CITY, Mo. — When the coronavirus pandemic first hit the U.S., sales of window coverings at Halcyon Shades quickly went dark. So the suburban St. Louis business did what hundreds of other small manufacturers did: It pivoted to make protective supplies, with help from an $870,000 government grant.
But things haven’t worked out as planned. The company quit making face shields because it wasn’t profitable. It still hasn’t sold a single N95 mask because of struggles to get equipment, materials and regulatory approval.
“So far, it has been a net drain of funds and resources and energy,” Halcyon Shades owner Jim Schmersahl said.
Many companies that began producing personal protective equipment with patriotic optimism have scaled back, shut down or given up, according to an Associated Press analysis based on numerous interviews with manufacturers. Some already have sold equipment they bought with state government grants.
Yet many manufacturers who answered the call have faced logistical hurdles, regulatory rejections, slumping demand and fierce competition from foreign suppliers. On April 1, Florida-based American Surgical Mask Co. became one of the latest to close.
“I’m just done with the fight,” CEO Matt Brandman told the AP.
After the initial scramble for PPE subsided, many industry newcomers faced difficulty selling products. Government agencies sometimes wanted huge quantities at tough-to-meet deadlines. Hospital systems tended to contract with established suppliers. Retail sales waned after every virus surge.
“At the end of the day, when everybody said they wanted American-made, nobody’s buying, not even the state,” said Tony Blogumas, vice president of Green Resources Consulting, a rural Missouri firm that received an $800,000 state grant but has sold only a few thousand masks. “We’re kind of upset about the whole situation.”
Missouri Gov. Mike Parson also is disappointed. His administration divided $20 million in federal COVID-19 relief funds among 48 businesses for the production of masks, gowns, sanitizer and other supplies. Parson hoped to seed a permanent field of manufacturers.
“I’m still a firm believer in that — that we need to be making PPE here in this state,” Parson said. “Unfortunately, a lot of entities went right back to where they were getting it before.”
Though federal stockpiles have been replenished, shriveling domestic production has raised concerns that state governments, medical facilities and others could again get stuck scrambling for gear during a future pandemic.
The AP identified more than $125 million in grants to spur production of pandemic supplies made to over 300 business in 10 states — Alabama, Hawaii, Indiana, Kansas, Louisiana, Maryland, Massachusetts, Missouri, New York and Ohio. It’s possible that grants were awarded in additional states, but there is no central clearinghouse to track them.
In November 2020, Alabama awarded one of the single largest grants — nearly $10.6 million from federal pandemic relief funds — to HomTex Inc. The company was to equip a new Selma facility to make 250 million surgical masks and 45 million N95 masks annually. The plant returned $1.8 million of the state grant and has yet to make anything due to a lack of customers.
“I can’t produce product that I can’t sell,” HomTex President Jeremy Wootten said.
Other companies also had trouble living up to political hype.
In October 2020, New York announced eight grants that then-Lt. Gov. Kathy Hochul, now the governor, said were “a model for how we build back better for the post-pandemic future.” Those included $800,000 for newly formed Altor Safety and $1 million for startup firm NYPPE.
But NYPPE’s equipment wasn’t ready until February 2021, by which time the market had changed, President Connor Knapp said.
So Knapp tapped the brakes on his plans. NYPPE still hasn’t sold any N95 masks because it lacks regulatory approval. It just recently scaled up production of surgical masks, after obtaining a U.S. Food and Drug Administration certification that came with its purchase of Altor Safety.
Some PPE manufacturers point to federal regulations as part of the reason for their struggles. Three-ply masks need FDA approval to be marketed for medical use — an important designation for building a long-term customer base.
That process can be time-consuming. Facing delays, Angstrom Manufacturing in Missouri ended up buying another business that already had FDA approval, President Chris Carron said. By then, it was fall 2021 — a year after it received a state grant.